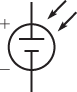Electricity consists of moving charge. When we think of the path for electricity, we think of wires most of the time. In metallic solids, electrons make up the charge that moves; however, the charge can also move through certain liquids and gases as well as space itself. In this section, you will learn the basic concepts of conductors, insulators, and semiconductors.
Conductors
Conductors are materials that allow the free movement of charge and can be composed of solids, liquids, or gases. Nearly all circuits use metallic solids such as copper or aluminum to provide a path for electricity. Wires and traces on pc boards are the most common form of the solid conductor for electrical signals. Wires are either solid or stranded.
Stranded wire is used for meter leads or in lamp cords where flexibility is important. No matter the size, a stranded copper wire has the same current capacity as solid copper wire as long as the gauge is the same in both.
For electronic circuits, copper is the most widely used material because it is an excellent conductor, can easily be drawn into wires, is corrosion resistant, and is cost-effective.
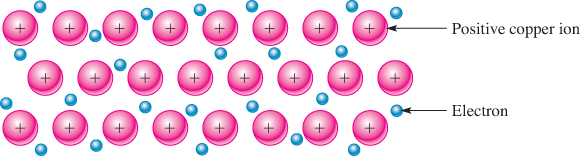
Copper is such a good conductor because of its atomic structure. Solid copper has a regular arrangement of positive ions (atoms or groups of atoms that have lost one or more outer shell electrons). This arrangement forms a basic metallic crystal. These electrons are free to move about but tend to distribute themselves to maintain overall electrical neutrality, as shown in Figure 1.
Figure 1: Metallic Bonding in Copper
The negatively charged electrons hold the positive ions of the metal together, forming the metallic bond. The “sea” of free electrons accounts for copper’s excellent electrical properties and is responsible for its metallic luster and other properties. Other solid metals have a similar structure to copper. Most are excellent conductors and have other properties that make them suited to specific electrical and electronic applications.
Gold, for example, can be drawn into extremely fine wires; it is noncorrosive, so it is used in switch contacts and as plating on plugs. Alloys (mixtures of two or more metals) also have many electrical and electronic applications. In the past, most solder used in electrical work was an alloy of lead and tin.
After July 1, 2006, the Restriction of Hazardous Substances Directive (RoHS) became effective and most manufacturers moved to lead-free solders composed of alloys of other metals. For most electronics work, the lead-free solders contain tin alloyed with bismuth, copper, silver or other metals. These soldiers are widely used for making solid connections on circuit boards, cables, and connectors.
In liquids, the moving charge is composed of positive and negative ions, never electrons. Materials known as electrolytes form ions in water solution and are good conductors. The three general categories of substances that form ions in water solution are acids, bases, and salts.
An example of a common acid that is used in lead-acid batteries is sulfuric acid, an example of a common base is sodium hydroxide (lye), and an example of a salt is ordinary table salt. In addition to water solutions of salts, molten salts can conduct electricity also. Ordinary table salt is composed of a crystalline structure of sodium (Na+) ions and chlorine ions (Cl−). When table salt is added to water, the crystal breaks apart and separate ions enter the solution. In solution, the sodium and chlorine ions are the charge carriers. The solution can conduct electricity due to the movement of these ions.
Gases can also conduct electricity when they are broken down into ions and electrons. Unlike liquid solutions, the charge carriers in a gas include electrons.
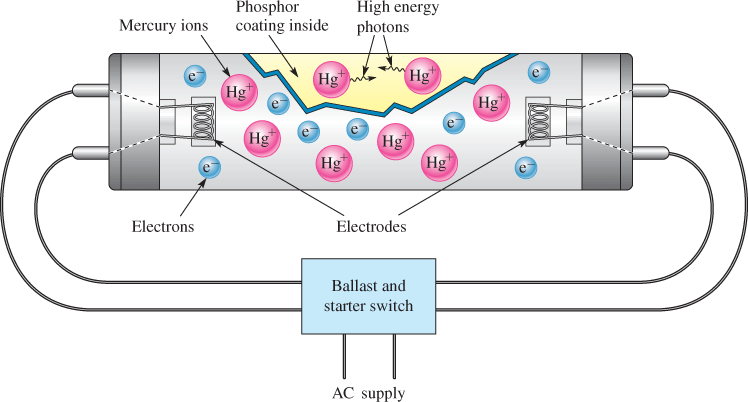
A good example of a gas conductor is the fluorescent lamp, shown in Figure 2. The tube contains low-pressure argon gas (not shown) and a small quantity of mercury. The instant the lamp is turned on, there are no free electrons or ions to allow current. An alternating voltage is applied to the electrodes, through the ballast, which controls the starting and regulates the current. The voltage on the electrodes heats the electrodes rapidly, causing electrons to boil off and start the process. These electrons move back and forth rapidly between the electrodes because of the voltage difference between them. The electrons ionize the mercury atoms in the tube, causing them to emit high-energy photons. Finally, the high-energy photons from the mercury interact with the phosphor coating on the inside of the bulb, emitting light composed of low-energy photons. All of this happens very rapidly in modern lamps, so rapidly that the light appears to come on almost instantaneously.
Figure 2: Fluorescent Lamp. In a fluorescent lamp, mercury ions emit high-energy photons, which are converted by the phosphor coating to visible light.
Insulators
Insulators are materials that prevent the free movement of charge; thus, they are considered nonconductors. While no material is a perfect insulator, many materials are such poor conductors that we classify them as nonconductors. The atoms in these materials tend to form bonds with other atoms that leave no “extra” electrons in the structure. The most common class of electronic insulating material is plastics. Other common solid materials used as insulators include ceramics, paper, and glass.
Plastics are widely used as the insulation layer on wiring. The insulating coating provides isolation from other conductors and is also important for protection against shock hazard. Sometimes a second coating is added to provide moisture or flame resistance to wiring. The type of insulation required depends on the application; for example, wiring that is used in solar applications needs to be rated for moisture and heat resistance. The maximum rated voltage, type of wire, AWG size, and manufacturer are all printed on the wire.
Wire Protection
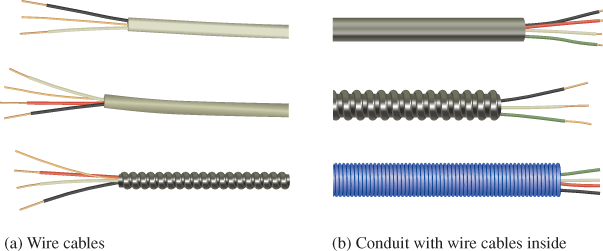
Two or more insulated wires are commonly bundled together within a plastic or armored sheathing to form a wire cable. See Figure 3(a). The individually insulated wires are color-coded for identification. Some local codes require armored cable or conduit (neither of which are necessarily made of insulating material) wherever wiring is exposed.
Conduit is essentially a pipe through which the insulated wires are run. It offers the best protection against wire damage. Also, conduit makes it easy to change or install new wires by simply pulling the wires or cables through the conduit without having to run new cable. Examples of metal and plastic conduits with wires run through them are shown in Figure 3(b).
Semiconductors
A semiconductor is a crystalline material that has four electrons in its valence shell and has properties between those of conductors (metals) and insulators (nonmetals). Chemically, silicon acts like a nonmetal, but it has metallic luster and electrical behavior closer to a metal. It is the most widely used semiconductor for electronics. Although pure silicon is not a good conductor, silicon can become a good conductor when certain impurities are added to it.
Figure 3: Color-Coded Wire Cables and Conduits
Two different types of impurities can be added to the crystal structure of the semiconductor.
One common impurity is phosphorous, which has five electrons in its outer shell. It is called an n-type impurity (n stands for “negative”) because four of the outer shell electrons bond with adjacent silicon atoms in the crystal, leaving an extra one available for conduction.
A different type of impurity is represented by boron, which has only three electrons in its outer shell. This is called a p-type impurity (p stands for “positive”). When it is introduced into the silicon crystal, one bonding position for the silicon is missing an electron. This missing position is called a hole.
Diode
When the p-materials and n-materials are manufactured together as one solid, some electrons on the n side move to fill holes on the p side, creating a region in the middle called the depletion region. Because of this depletion region, the resulting device is a good conductor if the voltage is applied in one direction and a good insulator if the voltage is applied in the other direction. Thus, current can pass in only one direction. This type of device is called a diode, a very important electronic device.
Because diodes allow current in only one direction, they are used in power supplies as a key component for converting ac to dc. The schematic symbol for a rectifier diode is shown in Figure 4(a) and (b).
When the voltage is applied with a polarity as shown in Figure 4(a), the diode conducts current (forward bias). When the polarity of the voltage is reversed as shown in Figure 4(b), the device blocks current (reverse bias). Another type of diode is the light-emitting diode (LED), so called because it emits light when current passes through it. The symbol is shown in Figure 4(c).
Photovoltaic Cell
The photovoltaic (PV) cell or solar cell is actually a type of diode designed specifically to allow sunlight to penetrate the semiconductor regions. Energy from sunlight releases electrons within the cell, which can drift across the depletion region, which, in turn, will cause a voltage to be generated. The schematic symbol is shown in Figure 5.
Figure 4: Symbols of a Semiconductor Rectifier Diode Showing Its Two Modes of Operation and a Light-Emitting Diode (LED)
Figure 5: Schematic Symbol of a Solar (Photovoltaic) Cell
Transistor
Many other electronic devices, such as the transistor, are constructed from semiconductors. The first successful transistor, invented at Bell Labs, was a semiconductor that used three layers of alternating p-type and n-type materials. This type of transistor called the bipolar junction transistor (BJT) is said to be a current amplifier because a small input current controls a large output current. Bipolar transistors are used in amplifiers, which are devices that increase the signal strength of a small signal such as the signal from a microphone.
Another type of transistor is the field-effect transistor (FET), which has become the dominant type of transistor. This type of transistor uses a voltage input to control the output current. It is particularly useful for switching applications, where the transistor acts like a voltage-controlled switch that can open or close on a voltage command.
The most important type of FET is the metal-oxide-semiconductor-field-effect transistor (MOSFET). Because of its importance in logic and in switching applications, the MOSFET is widely used in renewable energy systems. It is the primary component in integrated circuits, which form complex devices such as microprocessors and controllers for renewable energy systems.
A newer type of transistor called the insulated gate bipolar transistor (IGBT) combines the best attributes of the BJT and the FET for high-power switching applications. The IGBT combines the high-current capability of BJTs with the voltage control of a FET. The IGBT has made possible high-power devices that are much more efficient than older analog switching devices. For this reason, you are likely to encounter IGBTs when you work on high-voltage, high-current switching devices, which are common in large renewable energy systems.
Thyristor
The thyristor is another type of semiconductor device that is widely used. It is typically constructed of four semiconductor layers. Examples of thyristors include the diac, triac, silicon-controlled rectifier (SCR), and silicon-controlled switch (SCS). Thyristors can be used to control the amount of AC power to a load and are used in lamp dimmers, motor-speed controls, ignition systems, and charging circuits. These and other semiconductor components are studied in detail in courses dealing with electronic devices.
Review Questions
- What type of particle forms the structure of metallic solids?
- Why are metals good conductors?
- What is the name of liquids that are good conductors?
- What are two purposes for the insulated coating on wiring?
- What is a semiconductor?
- Explain what a diode does.
- What does PV stand for? What is a PV cell?
- What is the main purpose of an IGBT?
- What do the letters SCR stand for?
Answers
- Positive Ions
- Electrons are free to move within the structure and are not bound to any given atom.
- Ionic solutions
- Insulation is used for isolation from other conductors and to avoid the shock hazard.
- A crystalline material that has properties between a conductor and an insulator
- A diode is a device that allows current in only one direction.
- PV stands for “photovoltaic.” A PV cell (solar cell) is a type of diode that allows sunlight to penetrate the semiconductor regions, which causes atoms to release electrons. When electrons cross the PN junction, a voltage is generated.
- It is a transistor used for high-voltage, high-current switching.
- Silicon-controlled rectifier