An automotive battery is an electrochemical device (a device that uses both electrical energy and chemical energy to operate) that stores and produces electric current and voltage. A conventional automotive battery is also called a lead-acid battery because of the materials used in its construction.
A volt battery is made up of a group (or “battery”) of cells that generate an electric charge. A basic battery cell can be made by placing two dissimilar metal electrodes (metal plates that can donate and receive electrons) into an acid-filled container. Electrons are pulled from one electrode and attracted to the other, producing an electric current. See Figure 2.
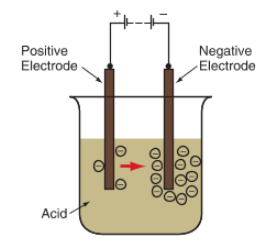
Figure 2. A basic battery cell can be made by placing two unlike metal electrodes in a jar of sulfuric acid. A chemical reaction occurs in which electrons flow between the two electrodes.
Battery Cell Construction
The parts of a basic battery cell include:
Positive plates—electrodes of lead peroxide (PbO2).
Negative plates—electrodes of finely ground or powdered lead (Pb).
Electrolyte—a solution of sulfuric acid (H2SO4) and water (H2O).
Separators—material that keeps the positive and negative plates from touching and creating a short circuit.
Battery plates are made of a stiff mesh grid coated with porous lead alloys. See Figure 3A. The chemically active material in the negative plates is sponge lead (lead that has been finely ground or powdered to increase its porosity).
Since the lead on the plates is porous, like a sponge, the sulfuric acid easily penetrates into the lead. This helps the chemical reaction and the production of electricity.
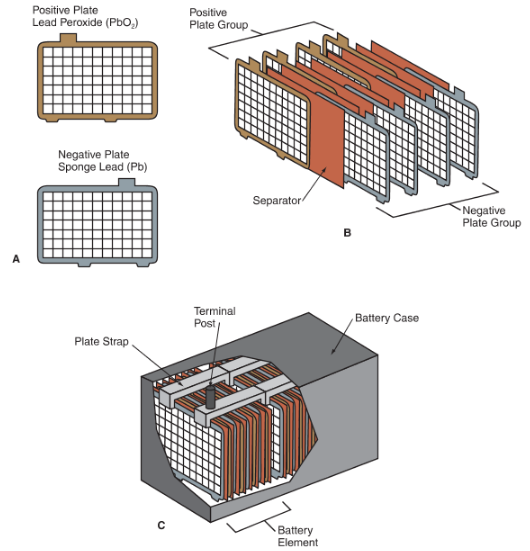
Figure 3.
A—Positive and negative plates. Notice the position of the tabs on the top edge. These tabs contact the plate strips to conduct electricity.
B—The plates in the positive and negative plate groups are interspersed, with separators to keep the plates from touching and shorting out.
C—A plate strap holds each battery element together. The plate straps placed end-to-end to conduct electricity from one cell to another. The terminal posts are usually formed onto the ends of the plate straps.
The active material on the positive plates is lead per-oxide. Calcium or antimony is added to the lead to increase battery performance and to decrease gassing (the formation of explosive hydrogen gas during the chemical reaction).
Several battery plates are needed in each cell to provide enough battery power for automotive use. See Figure 3B. A metal plate strap is used to join several negative plates to form a negative plate group. Another plate strap links the positive plates to make a positive plate group.
The connectors in each cell are placed in contact with the connectors in the adjacent cells so that electricity can be conducted along the length of the battery. See Figure 3C. The battery terminals (posts or side terminals) are constructed as part of the end connectors.
Separators fit between the battery plates to keep them from touching and shorting against each other. The separators are made of a porous insulating material that allows free circulation of the electrolyte around the plates.
The negative plate group, positive plate group, plate straps, and their separators make up a battery element; whereas, the battery cell contains these elements plus electrolyte. Refer again to Figure 3C.
The electrolyte in an automotive battery, often called battery acid, is a mixture of sulfuric acid and distilled water. This mixture is poured into each cell until the plates are covered to complete the functional battery cells.
Battery Case
The automotive battery case encloses the battery cells. It is usually made of high-quality polypropylene. The case must withstand severe vibration, cold weather, engine heat, extreme temperature changes, and the corrosive action of the battery acid. Dividers in the case form individual containers for each cell.
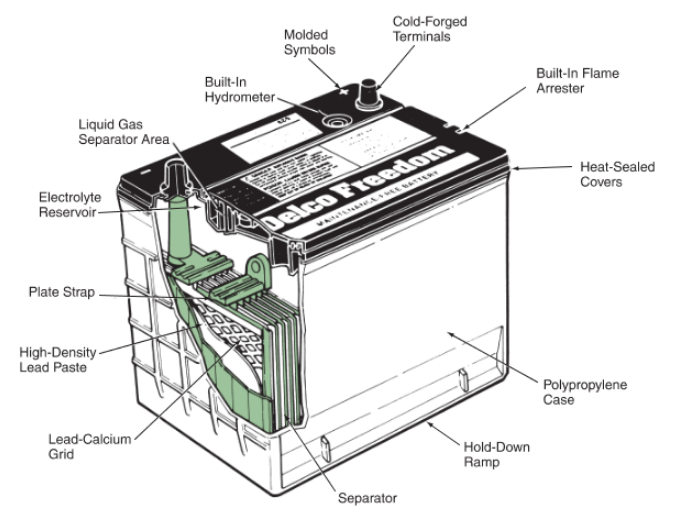
The battery cover is bonded to the top of the battery case. It seals the top of the case and provides an opening above each battery cell for battery caps or a cell cover. Refer to Figure 4.
Figure 4. Cutaway view of an automotive battery showing inside and outside components. (Delco)
Automotive Battery Terminals
Battery terminals provide a means of connecting the battery plates to the vehicle’s electrical system. They are usually formed as part of the end connectors. Two battery posts or side terminals can be used. Some large truck batteries have threaded posts, as shown in Figure 5.
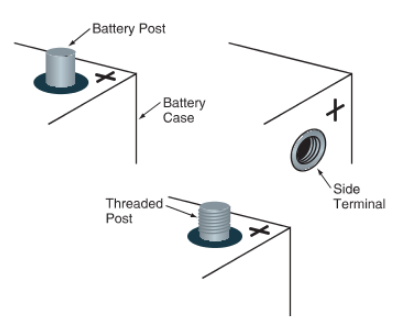
Figure 5. Basic terminal types. Side terminals are becoming more common because they resist corrosion very well.
Battery posts are round metal terminals sticking out of the top of the battery cover. They serve as male connections for female battery cable ends. The positive post is larger than the negative post. It may be marked with red paint and a positive (+) symbol. The negative post is smaller and may be black or green in color. It normally has a negative (–) symbol on or near it.
Side terminals are electrical connections on the side of the battery. They have female threads that accept a special bolt on the battery cable end. Side terminal polarity is identified by positive and negative symbols on the case.
Automotive Battery Charge Indicator
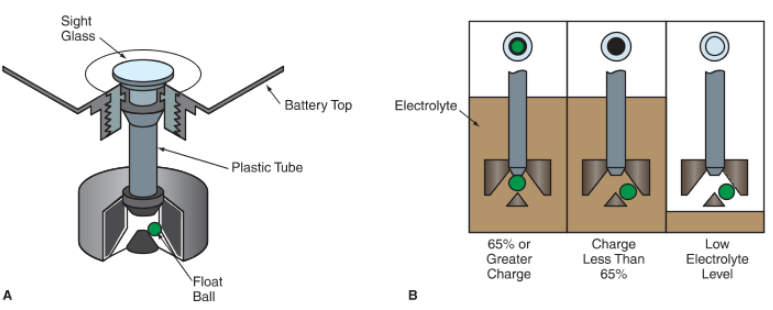
A battery charge indicator, or hydrometer, measures the specific gravity of the electrolyte in the battery as a measure of the general charge level of the battery. Refer again to Figure 4.
Specific gravity is the density of a solution relative to water, which has a density of 1. As the battery weakens, the specific gravity of its electrolyte becomes closer to 1.
The charge indicator changes color with changes in battery charge, as shown in Figure 6. For example, the indicator may be green when the battery is fully charged. It may turn black when the battery is discharged or yellow when the battery needs replacement.
Figure 6: A-The hydrometer is mounted in the battery cover. The small float ball floats higher when acid strength and state of charge are high. B—Operation of an automotive hydrometer.
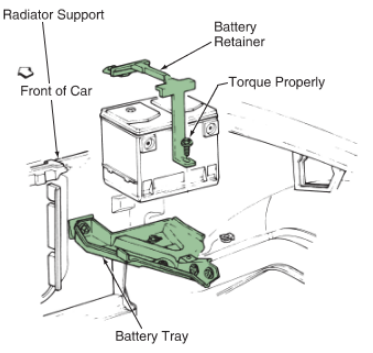
Battery Tray and Retainer
A battery tray and retainer hold the battery securely in place. They keep the battery from bouncing around during vehicle movement. It is important that the tray and retainer be in good condition and tight to prevent battery damage. See Figure 7.
Figure 7. The battery sits on the battery tray. The retainer holds the battery in the tray during vehicle movement. (Cadillac)
Automotive Battery Cables
Battery cables are large conductors that connect the battery terminals to the electrical system of the vehicle. See Figure 8A. The positive battery cable is normally red and fastens to the starter solenoid. The negative battery cable is usually black and connects to ground on the engine block.
In some vehicles, the negative battery cable has a body ground wire to ensure that the vehicle body is grounded, as shown in Figure 8B. If this wire does not make a good connection, a component grounded to the vehicle’s body may not operate properly.
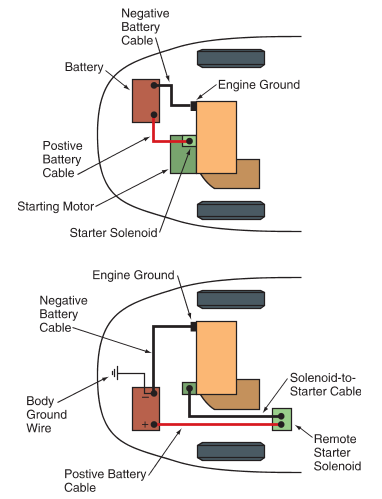
Figure 8. The automotive battery cable may be connected to the vehicle’s electrical system in slightly different ways, depending on the make and model.
Sometimes a battery junction block is placed between the positive battery terminal and the solenoid. This junction block allows other wires to make electrical contact with the positive battery cable to power the dash and accessories. See Figure 9. In other vehicles, the starter solenoid may serve the same function.
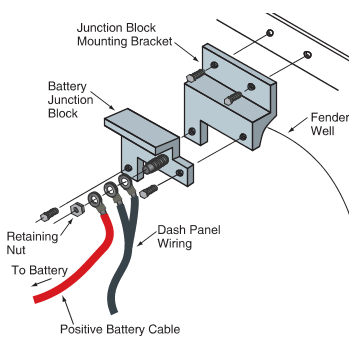
Figure 9. A junction block may be used so that other wires can obtain power from positive battery cable