This article covers the key differences between Conductor, Semiconductor, and Insulator on the basis of Conductivity, Resistivity, Forbidden Gap, Conduction, Band Structure, Current Flow, Band Overlap, 0 Kelvin Behavior, and Examples. The following table covers the key Differences between Conductor Semiconductor and Insulator.
- You May Also Read: Difference between Electric and Magnetic Circuits
| Characteristics | Conductor | Semi-Conductor | Insulator |
| Conductivity | High | Moderate | Low |
| Resistivity | Low | Moderate | Very High |
| Forbidden gap | No forbidden gap | Small forbidden gap | Large forbidden gap |
| Temperature coefficient | Positive | Negative | Negative |
| Conduction | Large number of electrons for conduction | Very small number of electrons for conduction | Moderate number of electrons for conduction |
| Conductivity value | Very high $\text{1}{{\text{0}}^{\text{-7}}}\text{mho/m}$ | Between those of conductors and insulators i.e. $\text{1}{{\text{0}}^{\text{-7}}}\text{mho/m}$ to $\text{1}{{\text{0}}^{\text{-13}}}\text{mho/m}$ | Negligible like $\text{1}{{\text{0}}^{\text{-13}}}\text{mho/m}$ |
| Resistivity value | Negligible; less than $\text{1}{{\text{0}}^{\text{-5}}}\text{ }\Omega \text{-m}$ | Between those of conductors and insulators i.e. $\text{1}{{\text{0}}^{\text{-5}}}\text{ }\Omega \text{-m}$ to $\text{1}{{\text{0}}^{\text{5}}}\text{ }\Omega \text{-m}$ | Very high; more than $\text{1}{{\text{0}}^{\text{5}}}\text{ }\Omega \text{-m}$ |
| Band structure | 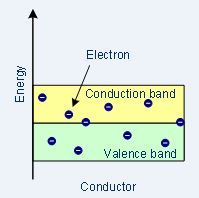 |
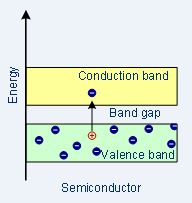 |
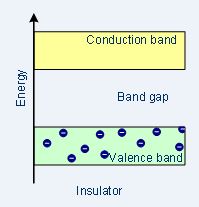 |
| Current flow | Due to free electrons | Due to free electrons and holes more than that in insulators | Due to free electrons but negligible |
| Number of current carriers at normal temperature | Very high | Low | Negligible |
| Band overlap | Both conduction and valence bands are overlapped. | Both bands are separated by an energy gap of 1.1eV | Both bands are separated by an energy gap of 6eV to 10eV |
| 0 Kelvin Behavior | Acts like a superconductor | Acts like an insulator | Acts like an insulator |
| Formation | Formed by metallic bonding | Formed by covalent bonding | Formed by ionic bonding |
| Valence Electrons | One valence electron in the outermost shell | Four valence electron in the outermost shell | Eight valence electron in the outermost shell |
| Examples | Copper, mercury, aluminum, silver | Germanium, Silicon | Wood, Rubber, Mica, Paper |
Difference between Conductors, Semiconductors, and Insulators on the Basis of Energy Bands
Conductors
In conductive materials, no band gaps exist so electrons move easily using a continuous, partly full conduction band.
Semiconductors
In semiconductor materials, the band gap between the conduction band and valence band is smaller and at normal temperature (room temperature), there is enough energy accessible to displace a few electrons from the valence band into the conduction band.
As temperature increases, the conductivity of a semiconductor material increases.
Insulator
In insulators, there is a large band gap between the conduction and valence band. The valence band remains full since no movement of electrons occurs and as a result, the conduction band remains empty as well.