Automotive batteries produce direct current. They have the potential to produce a tremendous amount of current for their size. Automotive batteries are very dependable, normally providing several years of trouble-free service.
Discharging and Charging
When electrons are flowing out of the battery, the battery is releasing stored energy, as shown in Figure 1A.
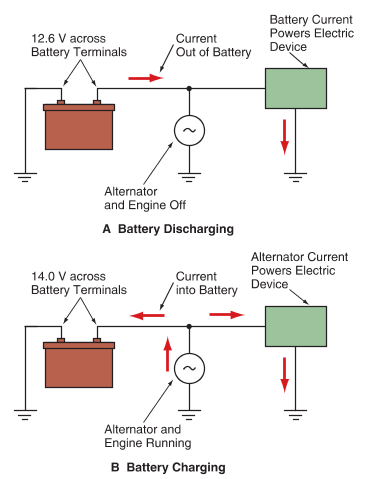
Figure 1.
A—When electrons flow out of the battery to power an electric device, chemicals in the battery are depleted.
B—When the alternator is running, the external voltage is slightly higher than the battery voltage, and electrons flow back through the battery to replenish the chemicals.
This process is called battery discharge. A discharge occurs when the battery converts chemical energy into electrical energy to operate an electric device. To charge a discharged battery, an external voltage slightly higher than the battery voltage must be applied to the positive battery terminal. This causes electrons back through the battery, restoring the chemicals in the battery to re-energize it. This charging process allows the battery to store chemical energy that can be converted back into electrical energy as needed. See Figure 1B.
Battery Cell Operation
Figure 2 illustrates the chemical reaction inside a battery cell. When the battery is discharging, oxygen in the positive plate combines with hydrogen in the sulfuric acid to form water and lead sulfate forms on the plates. As the battery continues to discharge, more of the acid changes to water and the charges on the plates become more similar. With enough discharge, the acid becomes weak and the plates both contain lead sulfate. The battery becomes discharged enough that it cannot provide the energy needed to function normally.
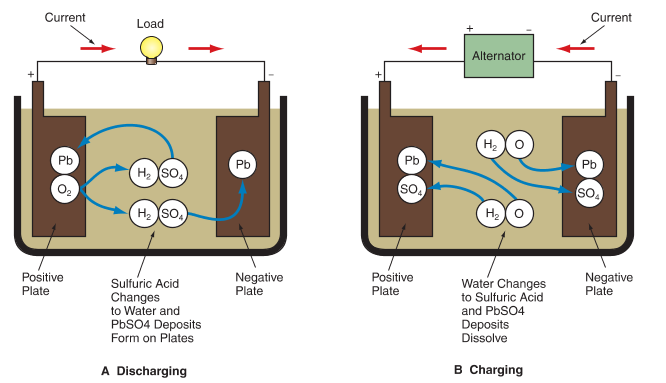
Figure 2. Chemical reactions that occur in an automotive battery.
A—As the battery discharges, the sulfuric acid changes to water and lead sulfate (PbSO4) is deposited on the electrodes. When all of the sulfuric acid has been changed to water, no further chemical reaction can occur, and the battery is dead.
B—Charging reverses the chemical reaction that happens during discharge. Water is converted back into sulfuric acid and lead sulfate is removed from the plates.
To charge a battery, electrons are forced back into the battery from the alternator or from a battery charger. For electrons to flow into the battery, the external voltage must be slightly higher than the total cell voltage.
During charging, the chemical reaction reverses. The sulfate on the plates is forced back into the electrolyte. The water in the electrolyte is split into hydrogen and oxygen. This chemical reaction returns the lead peroxide to the positive plate and lead to the negative plate. With enough recharging, the acid and plates return to their original state.
Battery Voltage
Open circuit (no load) cell voltage is 2.1 volts, often rounded off to 2 volts. Since the cells in an automotive battery are connected in series, battery voltage depends on the number of cells.
A 12-volt battery has six cells that produce an open circuit voltage of 12.6 volts (generally rounded to 12 volts). Most vehicles today use a 12-volt battery and a 12-volt electrical system. See Figure 3.
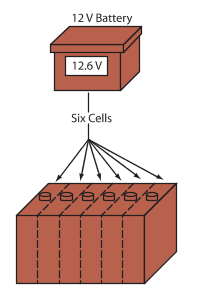
Figure 3. The automotive batteries used in late-model vehicles have six cells. Each cell produces 2.1 volts, so a battery generates a total of 12.6 volts
A diesel engine’s high compression ratio makes it difficult for the starting motor to crank the engine. Two batteries may be used on some vehicles with diesel engines to provide the high current for the starting motor. The two batteries are wired in parallel so the output remains at 12 volts (actually 12.6 volts). See Figure 4.
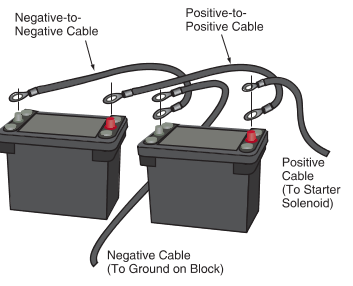
Figure 4. Two batteries may be connected in parallel to crank a compression ignition or diesel engine.
Battery Temperature and Efficiency
As automotive battery temperature drops, battery power is reduced. At low temperatures, the chemical reaction inside the battery slows down, and the battery cannot produce as much current as when it is warm. This affects the battery’s ability to start an engine in extremely cold weather. See Figure 5.
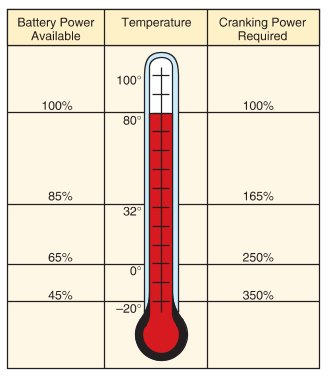
Figure 5. Automotive Battery power decreases as temperature decreases. This is part of the reason why engines crank slowly in cold weather.
Also, when an engine is cold, the motor oil in the pan is very thick. The oil is difficult to pump through the engine, and part friction is increased. This increases the amount of current needed to crank the engine with the starting motor.