Nuclear Energy
Nuclear energy derives its power from the enormous amount of stored binding energy in the nucleus of the atom, which is the energy that holds the nucleus of the atom together. By tapping this energy, a substance such as water is heated and converted to steam to drive a turbine and produce electricity.
Binding Energy per Nucleon
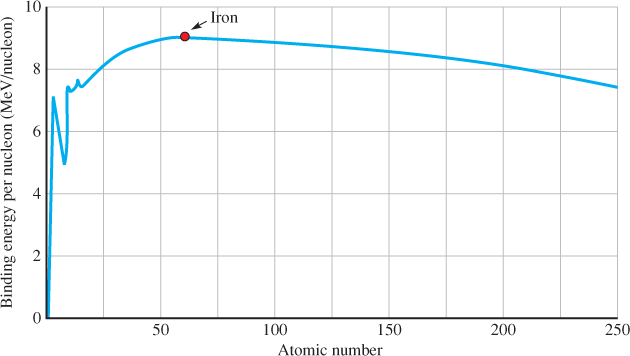
A nucleon is an atomic particle—either a proton or a neutron—found in the nucleus of an atom. Heavy atoms like lead (Pb) or uranium (U) have many nucleons, whereas light atoms, like hydrogen (H) and helium (He), have very few nucleons. The binding energy is the energy that holds the nucleus together. It is different for each atom and depends on the number of nucleons. Figure 1 is a plot of the binding energy in million electron volts (MeV) per nucleon. Notice that the highest binding energy per nucleon occurs with element 26, which is iron, with 56 nucleons. Above or below iron, the binding energy per nucleon drops.
Albert Einstein showed that mass and energy are directly related in his famous equation E = mc2 The graph in Figure 1 shows that the most stable nucleus is iron because it has the highest energy (least mass) per nucleon. If a heavier nucleus breaks apart, the “extra” mass is converted to energy, and this is a huge amount for a single atomic event, several million times greater than the energy released in a chemical reaction like burning methane.
The process of breaking a heavy nucleus into smaller fragments and releasing energy is called fission. All commercial reactors that are used for producing electricity are fission reactors and rely on breaking apart heavy atomic nuclei into fragments. The process of putting together (or fusing) light nuclei to produce energy is called fusion. Fusion is the ongoing process in the core of the sun that accounts for the prodigious amount of energy it produces.
Figure 1 Binding Energy per Nucleon
Nuclear Fission
As you can see from the binding energy curve, nuclear fission can release large amounts of energy. The problem is that only a very few materials can be broken down and release more energy than we put in, although most heavy elements can be broken apart if the energy of the incoming projectile is high enough.
In 1939, it was discovered that a specific isotope of uranium (235u) could be broken apart with low-energy neutrons (called thermal neutrons), and would release energy and immediately release additional neutrons. Natural uranium contains only 0.7% 235U to be useful for reactors, the uranium has to be enriched. Enrichment is a complicated process that involves separating 235U from natural uranium, which is mostly 238U. The amount of enrichment for a reactor varies, depending on the type of reactor, but is usually between 1% and 2%.
Neutrons emitted during the fission process are called prompt neutrons because they are emitted as the heavy nucleus breaks up, but they tend to have high energies, which are not efficient for continuing the fissioning process. These neutrons are reduced in energy by a moderator, which is a material that absorbs energy from fast-moving neutrons and slows them down. On average, 2.46 neutrons are emitted by fissioning of 235U. If each of these neutrons triggered another fission event, the process would grow exponentially and lead to an explosion. This type of reaction is called a chain reaction, which is a self-sustaining reaction. The reaction continues if at least one neutron triggers another fission event.
Figure 2 illustrates a nuclear fission chain reaction. Each fission reaction leads to additional neutrons that can continue the reaction and to additional fission fragments, which are the lighter nuclei left over from the fissioning. Most of the fragments are radioactive and produce radiation over varying half-lives (a half-life is the time required for one-half of the substance to decay).
Plutonium is another fissionable material used in certain types of reactors. Although plutonium occurs only in small amounts naturally, it can be made in reactors in a two-stage process from 238U during which it captures a fast neutron and emits an electron to become 239Pu, which can support a chain reaction.
In particular, a type of reactor called a breeder reactor is designed to produce plutonium, which could extend uranium supplies considerably because it can convert the otherwise unusable 238U into a fissionable fuel. Breeder reactors have not been used for generating power in the United States. One issue is that plutonium is particularly useful in nuclear weapons, so the widespread use of breeder reactors would greatly complicate issues of controlling nuclear weapons.
Figure 2 :A Fission Chain Reaction
Nuclear Reactors
All commercial reactors rely on nuclear fission, and they must be able to control the rate at which fissioning takes place. The chain reaction described previously would be almost impossible to control except for one important fact. Not all of the neutrons that are the result of fissioning are prompt (instantaneous) neutrons. A small fraction of the neutrons are emitted many seconds later by certain fission fragments. These delayed neutrons are critically important for controlling a reactor.
By carefully controlling the number of neutrons that are present in the core of a reactor, and hence the neutron multiplication factor, the power level can be adjusted up or down. Prompt neutrons produced from the fission reaction have a high probability of being lost and not contributing to the continuation of the reaction. If this were true for all the neutrons, the reaction would die out. A way around this problem is to slow down the prompt neutrons, producing thermal neutrons. This is done by mixing the fuel in reactors with a moderator, which is typically water or graphite.
To be an effective moderator, a substance needs to have a lightweight nucleus, as does hydrogen. Water is an effective moderator because each molecule contains two hydrogen nuclei H2O.
Types of Nuclear Reactors
Pressurized Water Reactor (PWR)
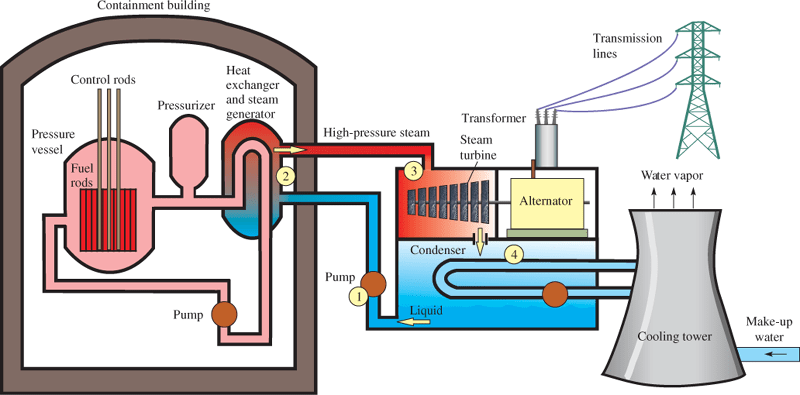
There are various types of reactors. A pressurized water reactor (PWR) is the most common type. In a PWR, the reactor fuel is loaded in rods called fuel rods, which are long thin tubes loaded with the fissionable material [typically enriched uranium oxide UO2]. The fuel rods are inserted in a pressure vessel, which is filled with water that is under high pressure to prevent it from boiling. Control rods are also in the vessel and contain a good absorber of neutrons, like cadmium (Cd) or boron (B). These rods can be moved in or out to control the reaction. The water in the vessel serves as both a moderator and a means to move hot water to a heat exchanger and eventually to a steam-driven turbine and an alternator.
Fast Breeder Reactor
A much more efficient reactor is the fast breeder reactor, which can use the much more abundant 235U as a fuel. This uranium fuel creates 239Pu and extends uranium fuel supplies for thousands of years. Many issues surround the use of the fast breeder reactor, including the use of the plutonium in nuclear weapons. Also, liquid water cannot be used as a coolant to keep high-energy neutrons in the system, so the more dangerous liquid sodium must be used instead. So far, fast breeder reactors have been used only in limited numbers, and some countries have shut down their breeder programs.
Small Modular Reactor (SMR)
A new type of reactor that is being developed is the small modular reactor (SMR), defined as a reactor that is less than 350 MW. SMRs will have a standardized design and will include passive safety procedures that allow shutdown without electrical power. Most of the reactor can be constructed in a shop rather than onsite.
One design has the reactor’s core located completely underground, beyond the reach of potential terrorists and able to absorb both earthquakes and tsunamis without the threat of overheating. Instead of large reactor cooling pumps, gravity induced flow will be used. The reactor will not need power from offsite locations to shut down.
Liquid-Fluoride Thorium Reactor (LFTR)
Another type of small modular reactor is the liquid-fluoride thorium reactor (LFTR). LFTRs are designed to convert thorium into uranium-233 (233U), which can then undergo fission. The reactor core is a molten salt core that is less complicated than that of a pressurized water reactor. Heat energy from the nuclear reaction is released into the fuel salt, which is then transferred through a heat exchanger to a coolant salt in a primary heat exchanger. The coolant salt, in turn, transfers its heat to a gaseous working fluid that drives a closed-cycle gas turbine, which produces electricity. Advantages to thorium-fueled reactors are that they create less hazardous waste and cannot be used to create nuclear weapons material.
A side benefit of an LFTR is that iodine-131 (131I), used to treat thyroid cancer, is produced during normal operation. Some other isotopes of medical interest are also produced. Currently, a thorium-fueled reactor is under construction in China.
No matter what technology is employed, the reactor is simply a means of super heating water to well above the boiling point and converting it to steam. The process from this point is similar to almost all thermal power stations, namely, converting the steam energy to mechanical motion and eventually electricity.
The turbine is a machine that converts the kinetic energy in a fluid into rotary motion. There are various types of turbines, including steam, wind, and water; they will be discussed throughout this text. The turbine is mechanically linked to an alternator, which produces electricity.
An alternator is simply an alternating current generator, and you will see both terms used interchangeably. Figure 3 shows a pictorial diagram of a typical pressurized water reactor for generating electricity.
Figure 3: Diagram of a Pressurized Water Reactor. The numbered points correspond to the steps in the Rankine cycle.
The portion of the PWR that holds the steam once the steam has been produced uses a standard Rankine cycle.
The Rankine cycle is a four-step, thermodynamic, closed-loop process that is used to convert energy in fluid to mechanical motion.
Briefly, the steps in the Rankine cycle are (1) pressurize the working fluid with a pump; (2) heat the working fluid to boiling or beyond boiling, thus converting it to a dry saturated vapor; (3) expand the dry vapor in a turbine, which extracts a large portion of the energy from the vapor and cools it, causing it to become a saturated vapor at reduced pressure; and (4) condense the wet vapor back to a low-pressure liquid by cooling it in a condenser at a constant temperature.