A voltaic cell consists basically of two different metal plates immersed in an acid solution. The action of the acid removes electrons from one plate and accumulates them on the other plate. In this way, a potential difference is produced between the two plates, and a current can be made to flow through an external circuit.
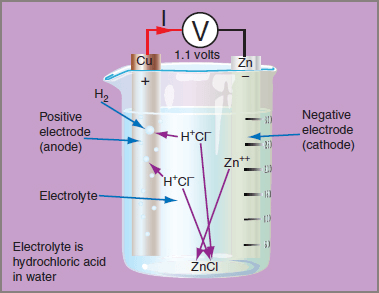
Figure 1 shows a simple voltaic cell consisting of two electrodes, one of copper and the other of zinc, immersed in a solution of dilute hydrochloric acid. The electrolyte need not be hydrochloric acid. Other acids such as chromic, acetic or sulphuric acid can be substituted — indeed, salt solutions such as common salt or copper sulphate can be used.
Figure 1 Voltaic Cell
In order to obtain a difference of potential between the two electrodes, only two conditions are necessary: the electrodes must be of different metals, and they must be immersed in an electrolytic solution comprising an acid, alkali or salt.
Voltaic Cell Construction
Because of early work in this area, these cells came to be known as galvanic or, more popularly, voltaic cells after two men (Luigi Galvani and Alessandro Volta).
The container is made from a non-metallic material (e.g. glass) that will not be affected by the acid. It must not be made of a conducting material, and care should be taken that the electrodes do not touch each other.
Voltaic Cell Working
Dissolving an electrolyte of hydrochloric acid (HCl) in pure water allows the acid to separate out into positive hydrogen ions (H+) and negative chlorine ions (Cl−). The chlorine ions combine with the zinc atoms, creating positive zinc ions (Zn++). This leaves free electrons on the zinc electrode, giving it a negative charge. The positive zinc ions combine with free chlorine ions, forming zinc chloride (ZnCl), a solid which sinks to the bottom of the cell.
Positive hydrogen ions move across to the copper electrode, where they combine with surface electrons from the copper electrode and become neutralized hydrogen atoms.
The hydrogen collects as bubbles that rise to the surface of the electrolyte and escape as free hydrogen gas. The removal of electrons from the copper electrode causes it to have a positive charge, and the cell has a potential equal to the difference between the negative charge on the zinc electrode and the positive charge on the copper electrode. This potential difference is usually about 1.1 volts.
This simple cell is not very practical, because the copper electrode becomes covered with hydrogen gas, preventing hydrogen ions from taking further electrons from the surface. This effect is known as polarization. Polarization increases the internal resistance of the cell, reducing the output voltage.
Local Action
The zinc used to form the negative electrode may contain small quantities of impurities that tend to form individual mini-cells by galvanic action within the main cell. Circulating currents are set up between the impurities and the electrode, causing the zinc to be eaten away even when the external load on the main cell is disconnected. To reduce this local action, it is necessary to use zinc electrodes that are as pure as possible.
Local action is not confined to cells, but can also occur in situations where different metals are in contact and moisture is present. The resulting action causes corrosion and destruction of the metals in contact.
Electrode Potentials
Two dissimilar metals in an electrolyte form a primary cell. The voltage across their terminals depends on the electrode potential of the metals used. Standard values have been recorded for a wide range of electrolytes and electrodes, with hydrogen taken as the reference value (0 volts). Other materials are given a value and arranged in order to indicate their potentials relative to hydrogen.
The electromotive force (EMF) between the electrodes in a voltaic cell is due to the chemical activity between the electrodes and the electrolyte. The electromotive force series (see Table 1) lists electrode potentials.
Table 1 Electromotive Force Series
| Metal | Electrode potential |
| Aluminium | −1.67 |
| Zinc | −0.76 |
| Chromium | −0.71 |
| Iron | −0.44 |
| Cadmium | −0.4 |
| Lead | −0.13 |
| Hydrogen | 0.0 |
| Copper | +0.34 |
| Silver | +0.8 |
| Gold | +1.42 |
For the cell shown in Figure 1, the voltage is the difference between the two electrode potentials given in Table 1. Copper has a more positive (higher) value than zinc, so it becomes the positive electrode (anode). The potential of the other electrode is subtracted from the anode value. Examples 1 and 2 show how this calculation is performed.
Example 1
What would be the EMF of the cell shown in Figure 1 where the electrodes are copper and zinc?
From Table 1: zinc is 0.76, copper is +0.34. The copper potential is more positive, so will become the positive electrode:
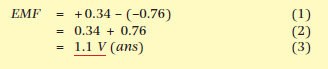
Example 2
What is the EMF of a cell similar to the cell in Figure 1 if the electrodes are silver and zinc?
From Table 1: zinc is 0.76, silver is +0.8.
The silver potential is more positive, so will become the positive electrode